Which Best Compares the Properties of Ionic and Metallic Substances
Bond energy is higher in covalent and ionic bonds than the metallic bonds. IONIC BOND COVALENT BOND METALLIC BOND.
Melting and boiling points of the covalent bond are low unlike the metallic bonds and ionic bonds which have higher.

. 1 an ionic bond 2 a covalent bond 3 a metallic bond 30 In the laboratory a student compares the properties of two unknown solids. This concept comparison is between chemical bonding of molecules and compounds. The other side has the bondcompound type Shop the Black Friday Sale.
There are three types of strong chemical bonds. Of electrons between the given pairs of atoms thereby. Help Ss contrast ionic and covalent bonding as well as metallic bonds and Van der Waals forces hydrogen bonds dipole-dipole London dispersion and Debye The Concept Comparison Routine is used help compare and contr.
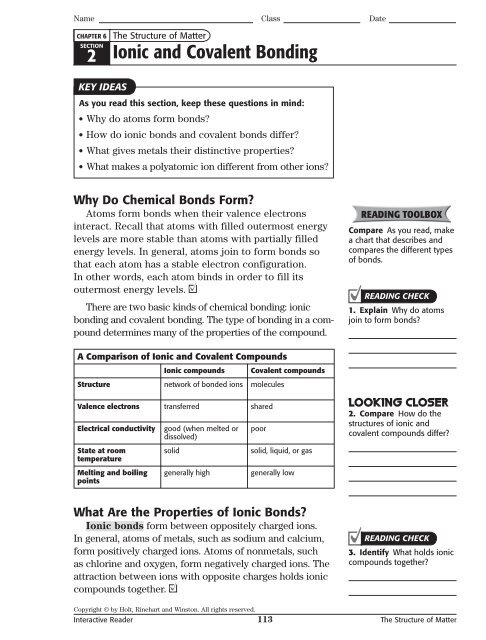
A metallic substance has a low melting point and an ionic substance has a low melting point. Electropositive element to an atom of a non-metallic. If you know the chemical formula of a compound you can predict whether it contains ionic bonds covalent bonds or a mixture of bond types.
The chemical compound formed due to mutual sharing. 92 Network Covalent Ionic and Metallic Solids YOU ARE EXPECTED TO BE ABLE TO. Occurs when 2 atoms share their valence electrons.
Ionic covalent and metallic. The bonds of metallic substances are composed of delocalized electrons the bonds of ionic compounds are composed of transferred electrons The bonds of metallic substances are composed of isolated electrons the bonds of ionic substances are composed of shared electrons. Relate the physical properties of non-molecular solids to the forces holding them together.
What best compares the properties of ionic and metallic substances. The chemical compound formed as a result of transfer. Nonmetals bond to each other via covalent bonds while oppositely charged ions such as metals and nonmetals form ionic bondsCompounds which contain polyatomic ions may have both ionic and covalent bonds.
Group of answer choices. Binding energy is higher than the metallic bond. This remains lower than 300 C.
The attraction of metal cationsatoms and delocalized electrons. Occurs during the transfer of electrons. Ionic compounds exist in the solid-state.
On various substances dissolved in water to qualitatively determine if a substance is an electrolyte or a molecular compound. At room temperature and normal atmospheric pressure covalent compounds may exist as a solid a liquid or a gas whereas ionic compounds exist only as solids. The results of his experiment are reported in the data table below.
However molecular compounds remain in all three states at standard temperature and pressure. In a metallic lattice the attractive forcesbonds between metal ions and the surrounding mobile sea of electrons are strong. Binding energy is higher than the metallic bond.
The bonds of metallic substances are composed of delocalized electrons and the bonds of ionic substances are composed of transferred electrons. Recap we have learnt 2 types of bonds exist between compounds Covalent Bonds - Electrons are shared Ionic Bonds - Electrons are Transferred - Balancing char. A metallic substance insulates heat and electricity and solid ionic substances conduct heat and electricity.
Because metallic bonding is rather fluid ie. Here are some differences. For ionic bonding the particles are oppositely charged ionsFor covalent bonding the particles are atoms which share pairs of electronsFor metallic bonding the particles are atoms which share delocalised electrons.
Table compares and contrasts the properties of ionic and covalent compounds. Properties of Ionic and Covalent Compounds Ionic and covalent compounds differ in their properties because the particles in each of these two compounds are held together by different types of chemical bonds. Binding energy is less than covalent and.
Bonding results from the delocalization of valence electrons across the metallic lattice metals tend to have lower melting points. Get 50 off Quizlet Plus through Monday Learn more. Classify non-molecular solids as either network covalent solids ionic solids or metallic solids.
This aspect of the lab activity allows the student to observe the properties of both ionic and molecular compounds in solution and requires that the student interpret results to. The melting and boiling point of molecular compounds is low as in comparison to ionic compounds. The boiling and melting point of ionic compounds is very high ie above 300C.
Of one or more electrons from the atom of a metallic. Ionic bonding occurs in compounds formed from metals combined with non-metals. Metallic bonds are malleable and ductile while covalent bonds and ionic bonds non-malleable and non-ductile.
One side has the characteristic. Covalent compounds Ionic compounds composed of simple molecules. Characteristics of ionic metallic and covalent bonds and compounds.
Substance A Substance B Melting Point low high Solubility in Water nearly insoluble soluble Hardness soft waxy crystals hard crystals. Certainly metals are malleable and ductile and are good conductors of heat and electricity whereas ionic solids are frangible and non-conductive and again this is another. The bonds of metallic substances are composed of isolated electrons and the bonds of ionic substances are composed of shared.
Strong attractive forcesbonds require a high temperature to be. Covalent and ionic compounds can be differentiated easily because of their different physical properties based on the nature of their bonding.
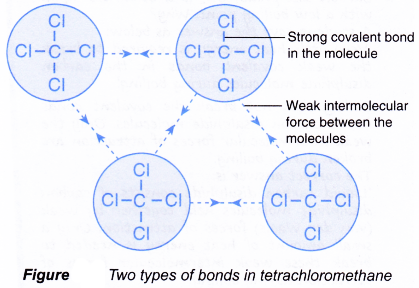
Properties Of Ionic And Covalent Compounds A Plus Topper
What Are The Differences And Similarities Between Ionic And Covalent Bonds Quora

Ionic Covalent And Metallic Bonds Differences And Similarities
Difference Between Ionic Covalent And Metallic Bonds Definition Formation Properties

Ranking Of Properties Of Solid Electrolytes 5 Best 1 Worst Download Scientific Diagram

Comparison Between Covalent And Ionic Compounds Introduction To Chemistry
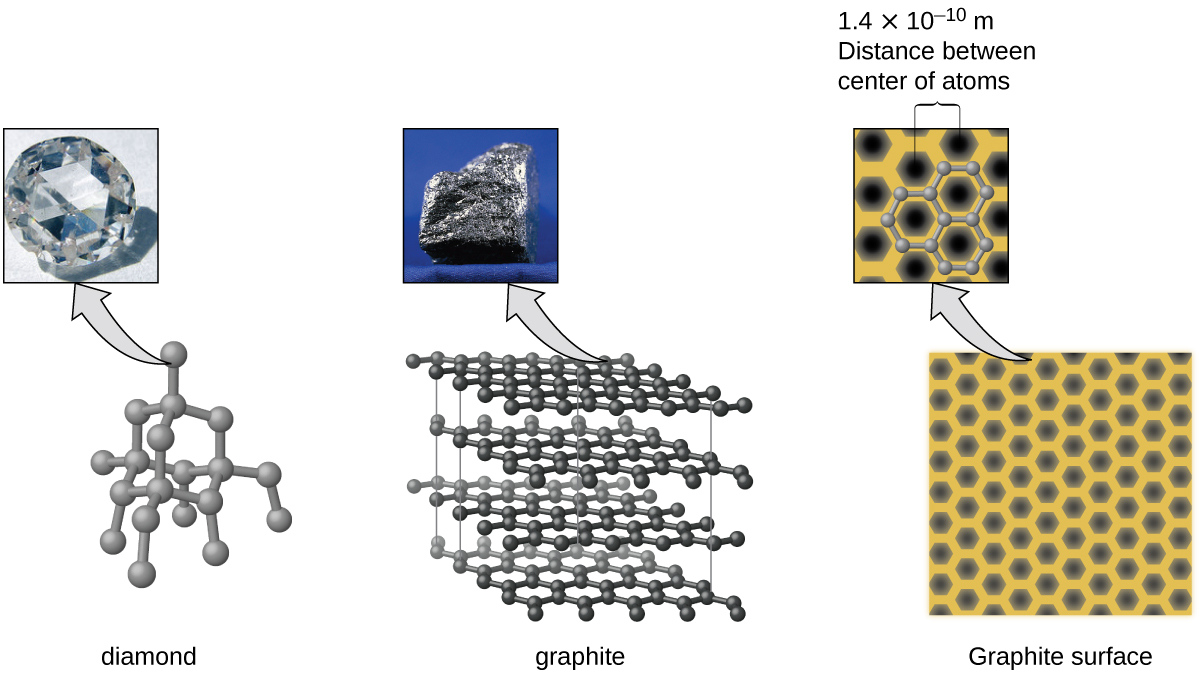
10 5 The Solid State Of Matter Chemistry
Difference Between Ionic Covalent And Metallic Bonds Definition Formation Properties

Ranking Of Properties Of Solid Electrolytes 5 Best 1 Worst Download Scientific Diagram
What Are The Differences And Similarities Between Ionic And Covalent Bonds Quora
Difference Between Ionic Covalent And Metallic Bonds Definition Formation Properties

Polarization Of Ionic Liquid And Polymer And Its Implications For Polymerized Ionic Liquids An Overview Towards A New Theory And Simulation Gao 2021 Journal Of Polymer Science Wiley Online Library

Ionic Covalent And Metallic Bonds Differences And Similarities
Challenges Free Full Text Ionic Mobility And Phase Transitions In Perovskite Oxides For Energy Application Html
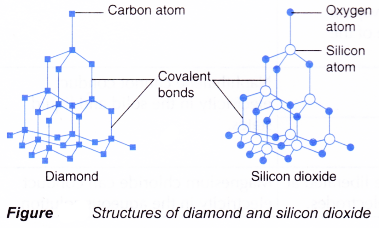
Properties Of Ionic And Covalent Compounds A Plus Topper

Ionic Bond Vs Covalent Bond Venn Diagram Shows The Similarities And Differences Between The Chemical Bonds Click Covalent Bonding Ionic Bonding Chemical Bond

Ionic Bond Vs Covalent Bond Venn Diagram Shows The Similarities And Differences Between The Chemical Bonds Click Covalent Bonding Ionic Bonding Chemical Bond
What S The Difference Between An Ionic Bond And A Covalent Bond Quora
Comments
Post a Comment